Mercury Barometer: Principle, Working, Advantages and Disadvantages
In this article, we will discuss detailed information about the mercury barometer along with its principle, working, advantages, and disadvantages.
What is Mercury Barometer?
A mercury barometer is an atmospheric pressure measuring instrument based on the displacement of mercury in a glass tube. It is also called the mercury manometer.
In short, a mercury barometer is used to measure the pressure exerted by the atmosphere at a given place.
Who Invented Mercury Barometer?
Evangelista Torricelli was the first who invented and discovered the principle of mercury barometers in 1644. He was also the first scientist to create a sustained vacuum and explain how to suction pumps actually work.
So, how Torricelli invented the mercury barometer?
The development of mercury barometers starts with the question of why “suction pumps only raise water to a height of 10 meters.” He stated the reason that it works on the force of vacuum but fails to explain why only up to 10 meters. The explanation lies in the atmospheric pressure.
On experimenting with the theory of atmospheric pressure, he started studying the behavior of mercury in variance to height. And finally, use his suction theory of vacuum to create a tube filled with mercury and vacuum. This led to the development of the first mercury barometer.
Because of the discoveries, physics terms such as “Torricellian tube”, “Torricellian vacuum” and “torr” (unit of pressure in vacuum) were named after him.
Facts: Torricellian works as an assistant in various experiments with Galileo.
Types of Mercury Barometers
Based on the adjustment of the glass tube, a mercury barometer can be categorized into six types.
1. Simple Mercury Barometer
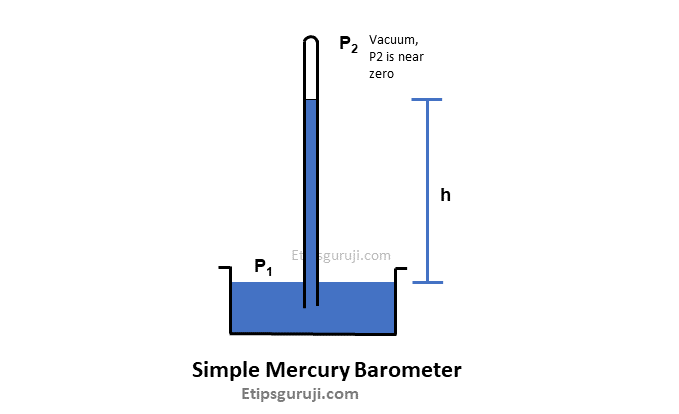
It has a vertical glass tube filled with mercury and has one end open and one sealed (like a test tube). After that, the vertical glass tube is inverted on a mercury-filled basis.
This results in the creation of a vacuum at the top of the glass tube. And the amount of liquid mercury displaces until it is equal to the weight of the air.
2. Differential Mercury Manometer
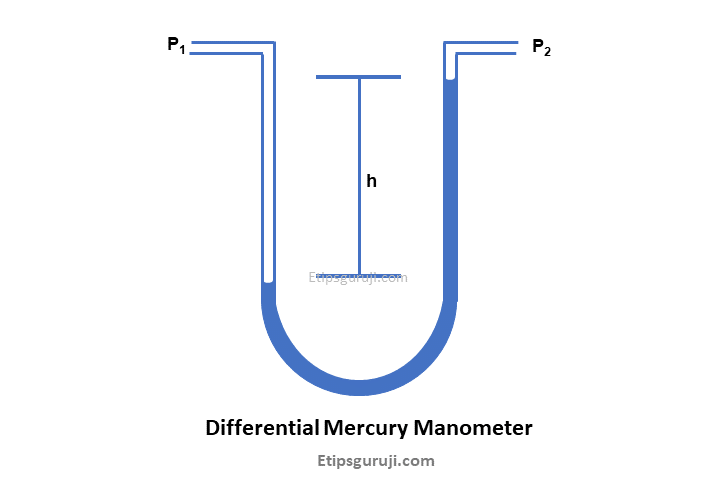
Though it works on the same principle as a simple one, it uses a U-shaped glass tube rather than a vertical one. And the amount of mercury displacement is analogous to atmospheric pressure.
3. Torricellian Barometer
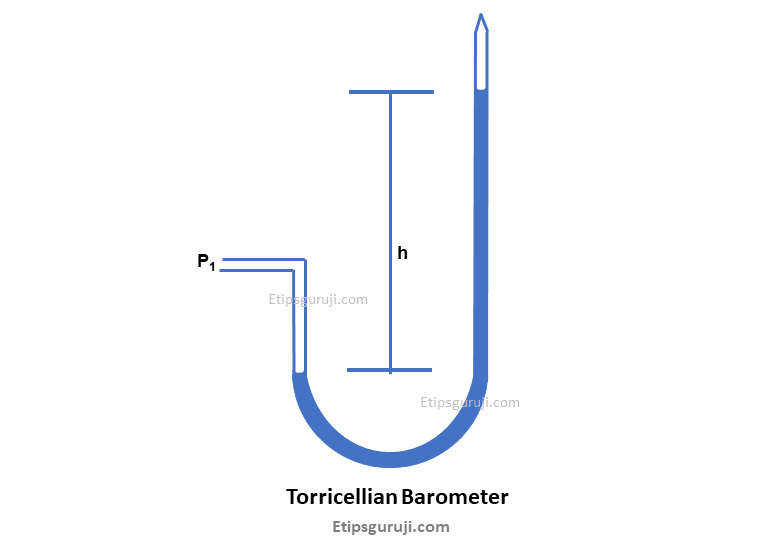
You can call it a combination of the simple and differential manometer. The glass tube has one straight vertical tube with a curved-edged glass. It resembles a u-shaped umbrella handle.
4. Siphon
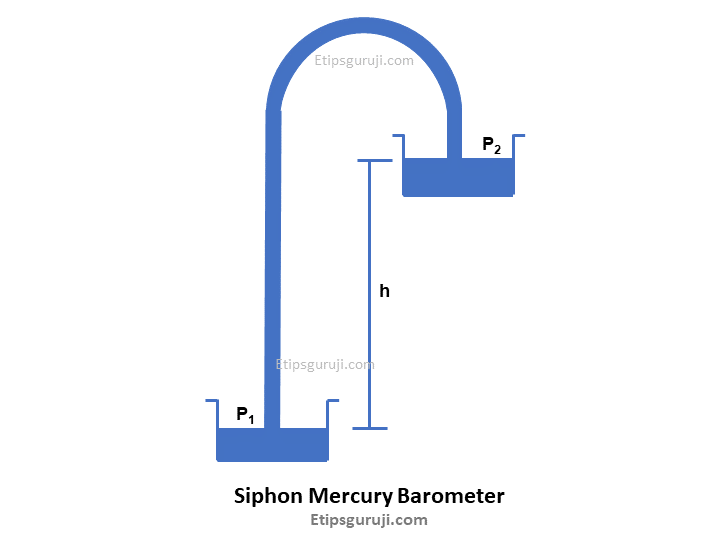
It uses a two-part mercury basin system. One basin is placed at a height while the other is placed at the bottom. Both mercury basins are connected through a tube bent to form two legs of unequal length to convey mercury from one basin to another.
5. Fortin Barometer
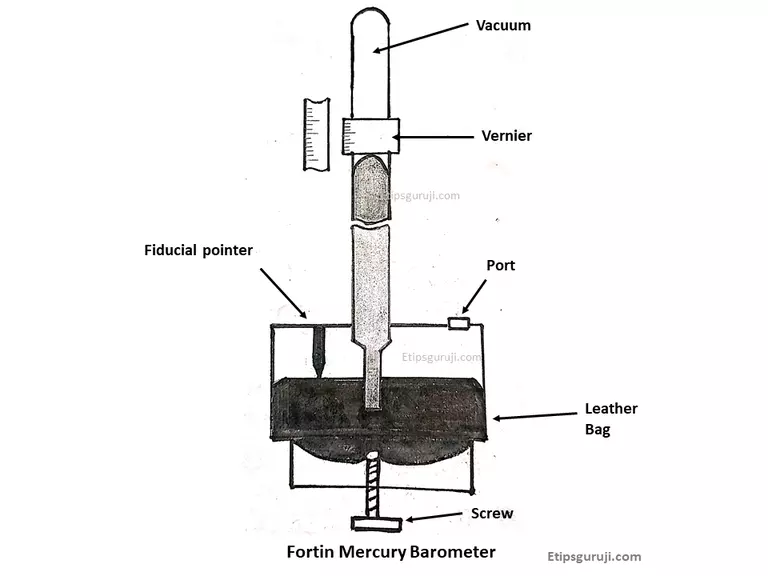
These barometers are used to measure the ambient pressure over the normal atmospheric pressure range. On their glass tube, it has a Vernier scale to measure the height of the mercury.
For calibration and adjusting mercury levels, it is done using a turning screen attached to a leather bag (adjustable cistern). They also have an attachment called Fiducial pointer to determine the zero of the vertical scale.
6. Kew Pattern Bench Barometer
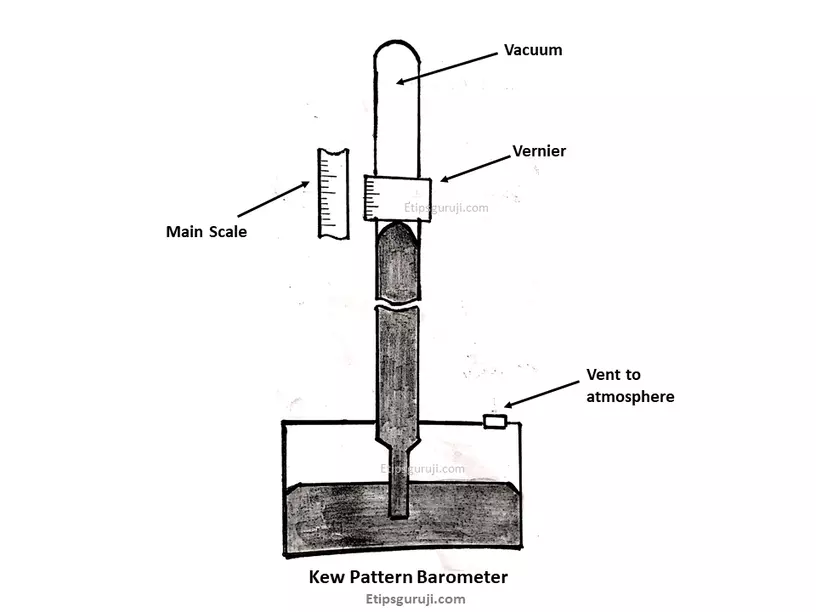
It is also called station barometer and structurally, it is similar to Fortin barometer except it has a fixed cistern. So, the mercury in the basin (cistern) can’t be adjusted. It is used to measure pressure from a few millibars to atmospheric pressure.
Why is Mercury Metal Used in Barometers?
Here are the four main reasons why mercury is used as pressure fluid in barometers:
- Density: The higher the density of a liquid, the lower its height. If we used water instead of mercury, it requires a tube of 10 m, which makes it inconvenient to use. Because mercury is 14 times denser than water, it can only reach 14 times the height.
- Easy to Read: Mercury is metal that’s why lustrous make it shiny and enable easy recording of small fluctuations.
- Stable Liquid State: It is the only metal that is liquid at room temperature. Plus, it has a high boiling point of 356.7 °C and a freezing point of -38.83 °C.
- Vapor Presence: Only a small amount of vapor is present at normal temperature in mercury, making the barometer more precise than other liquids.
Working Principle of Mercury Barometers
Mercury barometer works on the principle of balancing the atmospheric pressure with the displaced weight (volume) of mercury. Greater the mercury displaced by atmospheric air, higher is the atmospheric pressure.
Working: When one arm of the barometer is opened in unknown pressure, the mercury in the tube moves and displaces to a region and stops where the atmospheric pressure levels the liquid pressure at a certain height. The height is noted for calculating the atmospheric pressure.
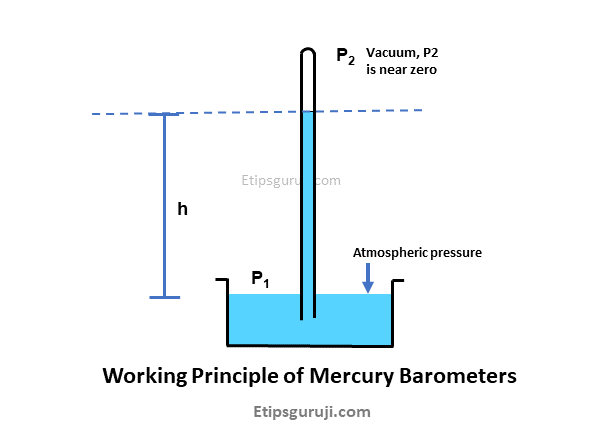
In general,
- Atmospheric pressure at the open side is the total pressure or also called absolute pressure (P1).
- Pressure due to the weight of a liquid of constant density is given by hρg, where ρ= density of mercury, g= acceleration of gravity, and h= difference between two liquid meniscus levels.
Therefore, Total pressure at the open end is given by:
Total Pressure at opening (P1) = Atmospheric pressure on vacuum side (P2) + Liquid pressure (mercury)
P1 = P2 + hρg
As per the given figure, there is a vacuum above point C and zero pressure. So, P2= zero, thus the total pressure (or absolute pressure) defines by
Total Pressure (P1) = hρg
As ρ (density) and g are constant so the total pressure changes are defined by the height of displaced liquid mercury.
Common pressure units for mercury barometric units are Millimeter of mercury (mmHg) or Inches of mercury (inHg).
Lastly, three factors that contribute to the accurate measuring of mercury barometers are:
- precision of measuring height
- Density of mercury
- Vapor pressure of mercury
How to Read the Mercury Barometer?
The accuracy of reading defines the correct atmospheric pressure. For reading the height there are two metrics currently available.
- Stick: In this type of representation, you can look directly at the top of the stick and compare the height of mercury in the glass tube with that of the engraved numbers near the column.
- Dial: The dial is also known as wheel or Banjo. You can simply watch the hand of the dial, which points to a particular number to read the mercury barometer.
Precaution While Reading Height in a Hg Barometer
- Ensure the height of the meniscus by lightly tapping on the mercury tube.
- By using a zero adjusting knob, adjust the level of mercury below zero.
- The height of a movable scale should be carefully set.
- The vernier scale can be used to record the height of the mercury.
- All note the temperature using the thermometer at the time of measuring pressure.
Factors Affecting Mercury Barometer Reading
Following are the factors that result in a change in Hg barometer reading:
- Gravity: Directly affecting the weight and height of mercury and atmospheric pressure.
- Height: Increase in height results in a decrease in atmospheric pressure, hence a decrease in mercury height.
- Temperature: With the increase in temperature, the atmospheric pressure decreases and so is the height of mercury.
- Humidity: A decrease in humidity would result in a fall of atmospheric pressure and height value.
- Sea level: Atmospheric pressure increases as we go down near the sea level and result in elevated height value, and vice versa.
- Weather– Upcoming weather changes the atmospheric pressure, which results in fluctuation of mercury levels even at normal locations.
Applications of Mercury Barometers
Following are some other uses of a mercury barometer:
- Mainly employed in science labs for demonstration purposes.
- For the measuring of atmospheric pressure for demonstration purposes including science labs, Aeronautics, meteorology, industrial, etc.
- It is also used to predict short-term weather forecasting.
- For preparation and calibrating aircraft’s altitude meters.
- Used for preparation of barographs (a barometer that records the barometric pressure over time in graphical form).
- For calibrating aneroid barometers
Advantages of Mercury Barometer
Following are some advantages of using a mercury barometer:
- Provides excellent statistics of precise and accurate atmospheric pressure measurement.
- Mercury used in the barometer has low vapor pressure due to which it does not evaporate easily.
- Mercury barometer does not require any expensive tool or equipment.
- Easy to construct in a science lab for demonstration purposes.
- No power for operation.
- Before taking the reading, it requires no adjustments.
Disadvantages of a Mercury Barometer
Some disadvantages of using a mercury barometer are:
- Toxic metal can cause mercury life-threatening poisoning.
- Toxic metal with a very thin layer of glass as protection.
- A small leak of mercury leads to hazardous health problems in the long run.
- Should need to properly vertically align to the surface to work properly.
- Higher chance of impurity with time because of exposed mercury basins in a simple mercury barometer.
- Requires a lot of space.
- Relatively outdated technology
- Not quite favorable for groundworks.
- Need a separate container for transport to avoid breaking glass.
- Commercially available mercury barometers are really expensive. They are more like showpieces.
[Table] Mercury Barometer Vs Aneroid Barometer
| Features | Mercury Barometer | Aneroid Barometer |
|---|---|---|
| Measuring Medium | Use liquid mercury to measure the weight displaced. | Use a small, flexible metal capsule of an alloy of beryllium and copper fitted with springs. |
| Measured by | Height of displaced mercury. | Expansion and contraction of metal. |
| Principle | Balancing between the weight of liquid to atmospheric pressure | Expansion and contraction of metals with change in atmospheric pressure. |
| Reading Values | Engraved numbers stick or Dial (banjo/wheel) | Dial |
| Chamber | A glass tube with one open and other sealed end | Sealed metal chamber with springs |
| Ease of Use | Unmanageable for daily use | Ideal for daily life. |
| Accuracy | Extremely precise and accurate. | Comparatively less accurate |
| Health Safety | Toxic liquid | Very safe to use |
| Construction Process | Can be manually built easily | Manufactured only through machinery |
| Size | Big and fragile | Smaller and compact |
| Usage | Need to be vertically positioned | Works in any position |
| Application | Only for demonstration purposes | Widely used and replaces mercury barometer |
Read More:
